Service
Clean Rooms (Pharma/GMP)
Turnkey classified suites—architecture, clean HVAC, interlocks & utilities engineered for ISO 5–8 and GMP compliance with full qualification support.
ISO classes
ISO 5–8 / EU-GMP
Modular panels
Flush doors, pass-boxes
Pressure cascades
Monitored & alarmed
Docs & PQ
DQ/IQ/OQ/PQ support
Scope
- User requirements & zoning maps
- Modular panels, covings & flush detailing
- Doors, view panels, interlocks & pass-boxes
- Clean HVAC with terminal HEPA / laminar modules
- Pressure cascade design & monitoring
- Flooring (ESD/vinyl/epoxy) & utilities (PW, drains)
- Electrical, lighting & controls
- Qualification: DQ, IQ, OQ, PQ with records
Deliverables
- Design package, BOQs & interlock diagrams
- As-built drawings & labeling
- Qualification protocols & reports
- O&M manuals, SOPs & training logs
Benefits
- Audit-ready, compliant facilities
- Robust particle & pressure control
- Cleanable, maintainable finishes
- Clear documentation trail
Built clean, validated clean
Corners, seams and flows that pass scrutiny.
Flush Detailing
Minimal ledges, continuous coving, sealed penetrations.
Controlled Interlocks
Airlocks & door logic protecting pressure cascades.
Qualification Support
From DQ to PQ with protocols, deviations & reports.
Technical specifications
- Powder-coated / SS modular panels with flush view panels
- Radius covings, chemical-resistant sealants
- Pass-boxes (dynamic/static) with interlock logic
- Air change targets by class; terminal HEPA & laminar modules
- Room differential monitoring & alarms
- Sampling points & PAO testing capability
- DQ/IQ/OQ/PQ protocols & reports
- Corrective actions & traceability
- O&M manuals, SOPs & training records
Gallery
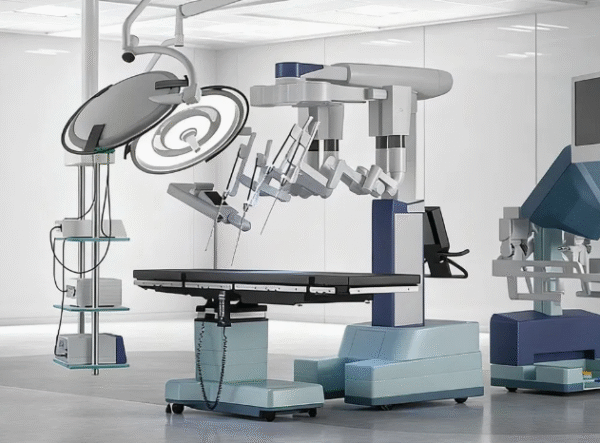

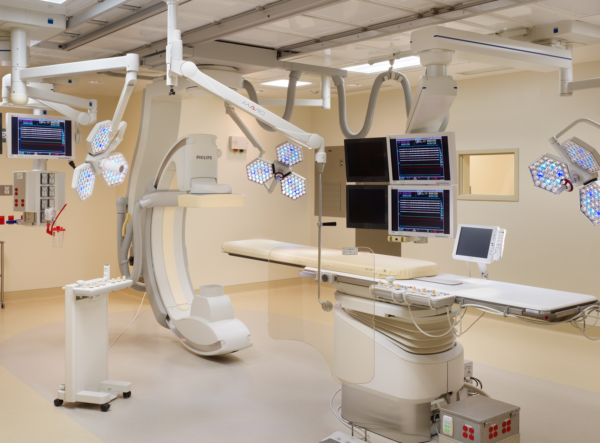
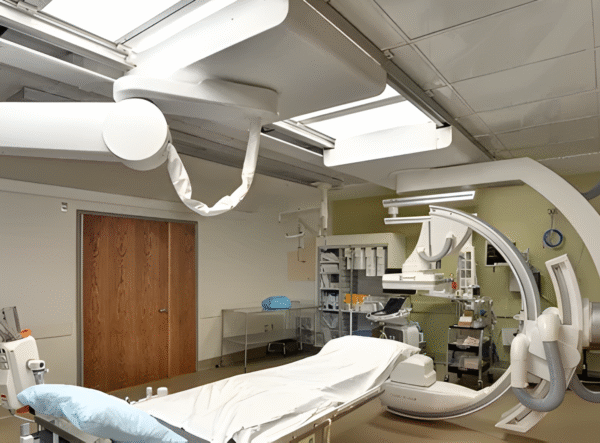

Planning a cleanroom suite?
We’ll design the cascade and validate rigorously for your process.

